by A.P.
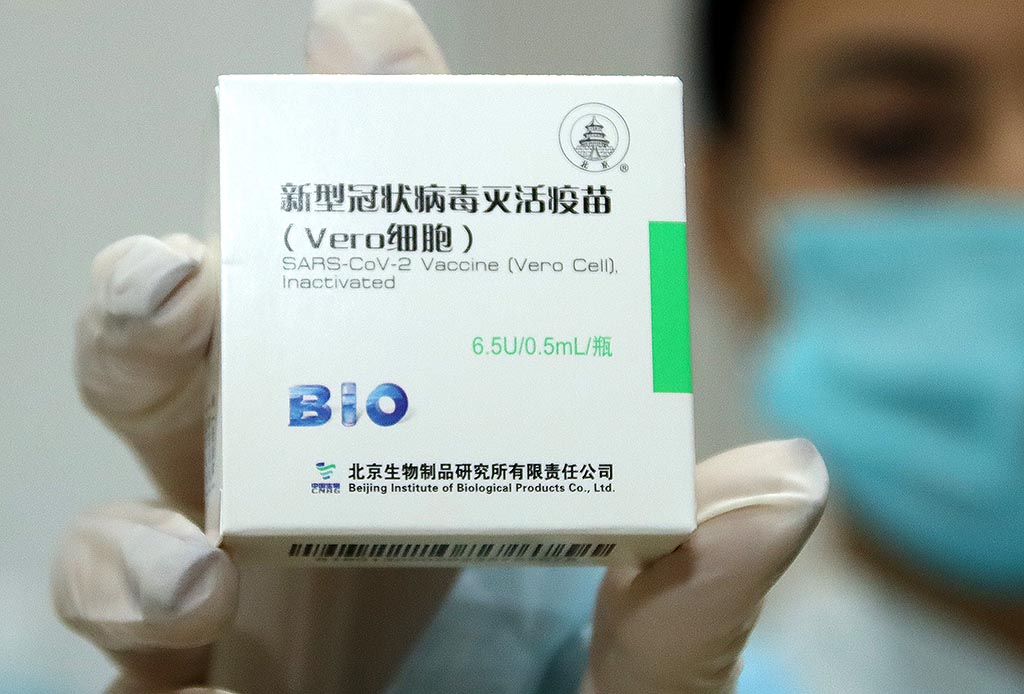
Hungary’s National Food and Drug Safety Institute gave an emergency approval to the Chinese Sinopharm COVID-19 vaccine, which could speed up the country’s immunization process, health officials said over the weekend.
National Chief Medical Officer Cecília Müller said on Sunday that the Chinese vaccine is formulated to contain a complete, inactivated virus, eliciting a strong immune response. She noted that Hungary’s own vaccine development also chose this technology, meaning that if the virus changes, this technology will later allow more room for maneuver.
Four batches of the Sinopharm vaccine have so far arrived to Hungary and, following emergency approval, each one must be released by the National Public Health Center on a batch-by-batch basis. In all cases, the documentation sent by the manufacturer should be compared with the results of an independent study, the chief medical officer said.
Meanwhile, Hungary is seeing a fresh rise of coronavirus cases, indicative of a third wave. According to the latest official data, 47 patients died and another 2,912 citizens were diagnosed with the coronavirus infection. The government portal wrote that the number of active infected people is 82,103, with 4,233 people in hospitals and 366 of whom are on ventilators.